Corrective and Preventive Actions (CAPA)
| Line 2: | Line 2: | ||
=Abstract= | =Abstract= | ||
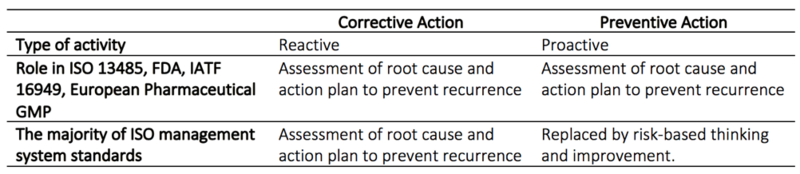
| − | Corrective and Preventive Actions (CAPA) are procedures designed to handle nonconformity and other undesirable situations. In this context is corrective actions is defined as actions set in motion to eliminate an occurred nonconformities or unwanted situations, and preventive actions as actions set in motion to eliminate potential nonconformities or unwanted situations <ref name="CAPAFDA">Rodríguez-Pérez, José American Society for Quality 2011, CAPA for the FDA Regulated Industry</ref>. | + | Corrective and Preventive Actions (CAPA) are procedures designed to handle nonconformity and other undesirable situations. In this context is corrective actions is defined as actions set in motion to eliminate an occurred nonconformities or unwanted situations, and preventive actions as actions set in motion to eliminate potential nonconformities or unwanted situations <ref name="CAPAFDA">Rodríguez-Pérez, José American Society for Quality 2011, CAPA for the FDA Regulated Industry</ref>. CAPA is a mandatory part of the Quality Management System (QMS) for any pharmaceutical or medical device manufacturer reporting to the U.S Food and Drug Administration (FDA). CAPA is also an integrated part of ISO:13485 and Good Manufacturing Practice (GMP) for medical products. The FDA defines the purpose of a CAPA procedure as: collecting and analyzing information, identifying and investigating product and quality problems, and taking appropriate and effective corrective and/or preventive action to prevent their recurrence <ref name="FDA">FDA website Corrective and Preventive Actions (CAPA) https://www.fda.gov/corrective-and-preventive-actions-capa#page3.</ref> |
| − | + | ||
| − | + | ||
| − | CAPA is a mandatory part of the Quality Management System (QMS) for any pharmaceutical or medical device manufacturer reporting to the U.S Food and Drug Administration (FDA). CAPA is also an integrated part of ISO:13485 and Good Manufacturing Practice (GMP) for medical products. The FDA defines the purpose of a CAPA procedure as: collecting and analyzing information, identifying and investigating product and quality problems, and taking appropriate and effective corrective and/or preventive action to prevent their recurrence <ref name="FDA">FDA website Corrective and Preventive Actions (CAPA) https://www.fda.gov/corrective-and-preventive-actions-capa#page3.</ref> | + | |
| − | + | ||
The purpose of this article is to give the reader an overview on how to perform a CAPA and which risk to be aware of. In this article a 7 step framework is presented. The steps include 1) Identification; 2) Evaluation; 3) Investigation; 4) Analysis; 5) Action Plan; 6) Implementation; 7) Follow-up. | The purpose of this article is to give the reader an overview on how to perform a CAPA and which risk to be aware of. In this article a 7 step framework is presented. The steps include 1) Identification; 2) Evaluation; 3) Investigation; 4) Analysis; 5) Action Plan; 6) Implementation; 7) Follow-up. | ||
Revision as of 18:03, 21 February 2021
Contents |
Abstract
Corrective and Preventive Actions (CAPA) are procedures designed to handle nonconformity and other undesirable situations. In this context is corrective actions is defined as actions set in motion to eliminate an occurred nonconformities or unwanted situations, and preventive actions as actions set in motion to eliminate potential nonconformities or unwanted situations [1]. CAPA is a mandatory part of the Quality Management System (QMS) for any pharmaceutical or medical device manufacturer reporting to the U.S Food and Drug Administration (FDA). CAPA is also an integrated part of ISO:13485 and Good Manufacturing Practice (GMP) for medical products. The FDA defines the purpose of a CAPA procedure as: collecting and analyzing information, identifying and investigating product and quality problems, and taking appropriate and effective corrective and/or preventive action to prevent their recurrence [2]
The purpose of this article is to give the reader an overview on how to perform a CAPA and which risk to be aware of. In this article a 7 step framework is presented. The steps include 1) Identification; 2) Evaluation; 3) Investigation; 4) Analysis; 5) Action Plan; 6) Implementation; 7) Follow-up.
The context of CAPA
Project management
Regulatory authorities
The 7 steps of CAPA
Step 1: Identification
Step 2: Evaluation
Step 3: Investigation
Step 4: Analysis
Step 5: Action plan
Step 6: Implementation
Step 7: Follow-up
CAPA Report and documentation
Limitations
Critically reflect on the tool/concept/theory and its application context. What can it do, what can it not do? Under what circumstances should it be used, and when not? How does it compare to the “status quo” of the standards – is it part of it, or does it extent them? Discuss your article in the context of key readings / resources provided in class. Substantiate your claims with literature
Other relevant Wiki articles
How to create a working CAPA team: Roles and responsibilities in project team.
How to control the scope of the CAPA project: Project Scope Management.
How to ensure control of the CAPA proces: Project Control.
How to improve your CAPA processes: Lessons learned.
Annotated Bibliography
Provide key references (3-10), where a reader can find additional information on the subject. The article MUST make appropriate references to the and reference material provided in class – either incorporating it as a source, or critically discussing aspects that are missing from it but covered by this article. Summarize and outline the relevance of each reference to the topic (around 100 words per reference). The bibliography is not counted in the suggested 3000 word target length of the article.
References
- ↑ Rodríguez-Pérez, José American Society for Quality 2011, CAPA for the FDA Regulated Industry
- ↑ FDA website Corrective and Preventive Actions (CAPA) https://www.fda.gov/corrective-and-preventive-actions-capa#page3.