Project Management in pharmaceutical industry
(→Approaches in R&D project management) |
(→Drug development stages) |
||
| Line 53: | Line 53: | ||
| − | The drug development basically consists of a sequence of phases in which each phase has a specific purpose and studies associated. It takes many years and it is therefore necessary to have a clear idea of all the steps,tasks and stakeholders involved in each of them. | + | The drug development basically consists of a sequence of phases in which each phase has a specific purpose and studies associated. It takes many years and it is therefore necessary to have a clear idea of all the steps,tasks and stakeholders involved in each of them.<ref name="drugDevelop" /> |
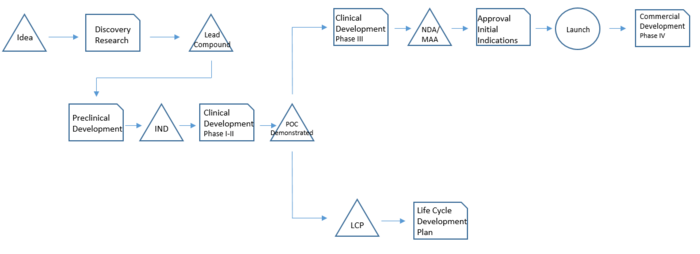
[[File:DrugDevelop.PNG|700px|thumb|right|Drug development phases. Inspired by "Drug development project management".]] | [[File:DrugDevelop.PNG|700px|thumb|right|Drug development phases. Inspired by "Drug development project management".]] | ||
Revision as of 21:21, 26 February 2019
Developed by Alberto Tognon
Contents |
Abstract
Project management is a discipline that can be applied to all industries, regardless of the product or service they are designed to deliver. Beyond its basic application across various industries, project management has tremendous value when effectively implemented to significantly increase the success of the product or service being delivered.
In the pharmaceutical industry, project management in R&D plays a big role in managing such a complex context due to unique regulatory, compliance and quality related needs of the industry. Pharmaceutical companies are now facing a new phase in which there are enormous challenges in the long product-development process. Delivering a new product to the market safely, quickly and cheaply is the best way for them to be successful. But an increasing competition, more stringent regulatory requirements and increasing costs for R&D are giving pharmaceutical firms even more challenges and the need to restructure their strategies trying to be more agile, and reduce the internal costs. Efficiency in all the aspect of business are thus fundamental.
In general, it takes 10 to 15 years to launch a new product in the market.[1] The activities related to the launch require coordination between many different stakeholders both internally as R&D, regulatory, legal, finance, supply chain, sales, and marketing, and externally with partners . It is therefore really important to get these activities coordinated in an efficient way and have transparency in all the steps. During this period of uncertainty a lot of things can happen and this makes the planning part of the project even more difficult. The company then has to take decision and adjust the plans along the process. It is considerably important to evaluate all the threats, and the risks must be treated in an effective and timely manner. The complexity would otherwise increase and the project becomes cost prohibitive.
This article defined uncertain and identifies challenges in project management in the pharmaceutical research and development, describing the critical factors, different approaches, key roles and helps to identify ways of dealing with these challenges.
Challenges in R&D Project Management
Project Management in pharmaceutical R&D represents a key element which knowledge is a must in order to deliver to the market new product in an effective, economic and timely manner. It generally takes 10 to 15 years to launch one new product to the market.[1] During this time it is required a lot of coordination between different stakeholders that usually have different priorities and backgrounds. The alignment throughout all the chain, from the initial idea to the sales, therefore needs a systematic and formalized project management approach for coordination, planning, scheduling, overseeing in the execution, and risk management. Moreover, the pharmaceutical industry is one of the industry that invest the most in research and their research and development activities are extremely expensive and highly risky. According to EFPIA[2] in 2018, the US pharmaceutical industry invested as much as $55.4 billion in research and development. This amount represents the 15.0% of the net sales. This huge expenditure in research and development force the pharmaceutical companies to make great effort to tackle down costs and time. Then, due to the high competition to arrive first in the market and the stringent regulatory requirements they have to take the risk of running multiple products-development projects in parallel, making the management even harder in focusing on any single product.
Uncertain in the pharmaceutical industry
Resource & development projects contain a lot of uncertain. In fact, just few new projects that are initiated greatly exceeds the number successfully completed. Just some drugs of the ones developed result in a successful marketing application. It is estimated that for every 5,000 to 10,000 molecules screened for biological activity, only 5 will go pass to clinical development and just one will get regulatory approval and enter in the market – this means a really low hit rate that is estimated to be around 0.02% to 0.01%.[3] This happens because new molecules and drugs development may be started based on untested hypothesis that might revel some threats due to unacceptable aspects of its profile, against what very little can be done to rectify the situation despite the money and the time already spent on it. Moreover the length of the project itself raise the probability of encountering unknown and unforeseeable risks that can result in turbulence in the project that can be incontrollable. Regulatory requirements are changing and becoming tighter, impacting product development - this might let a project fail or a change of direction on it with subsequent delays .
Many challenges arise while approaching a new project in R&D. Since the really beginning there are some strategic considerations that need to be addressed. Besides the potential therapeutic utility of the new drug, the project must also have commercial value and fit within an overall R&D strategy. The projects are therefore analyzed on external and internal considerations. External considerations include:
- the drug is first and/or best in class;
- Resource capacity and capability;
- there is a high market potential and growth opportunity.
Internal considerations include:
- Having in-house scientific expertise and know how;
- Resource capacity and capability;
- Budgetary constraints that will guide trade-offs.
Once these strategic considerations have been made and there is alignment between the all landscape, other elements must be analyzed. Considering the complexity and input requirements from various disciplines, the task to build the development plans and Life Cycle Plans is usually assigned to multi-functional asset planning team consisting of representatives from all the departments within the company. This helps to guarantee the involvement of all the functions of the company and allows the following advantages:
- Ensure alignment between R&D, commercial development and business units;
- Provides a common, unified strategy;
- Motivates people that are aiming for the same goals and objectives;
- Ensures the transparency of the Life Cycle Plan across the organization;
- Ensures consistency in initial development and life cycle planning processes.
All these elements are interdependent between each others and they all need to be considered as a whole thing, because lacking in just one of them might bring to the failure of the project. The project management team has therefore to take into consideration all these factors while starting a new project.
Drug development stages
"The drug development can be described as a continuous undertaking of tests, experiments, and studies to determine, define, and document the safety, efficacy, and quality of a particular chemical or biological compound with the ultimate goal to obtain approval from regulatory authorities to market the drug"[4]
The drug development basically consists of a sequence of phases in which each phase has a specific purpose and studies associated. It takes many years and it is therefore necessary to have a clear idea of all the steps,tasks and stakeholders involved in each of them.[4]
Discovery Research: The process starts with the identification of a molecule that revels some potential and that meets the strategic considerations – meaning it has both commercial and medical value potential. This molecule has therefore to follow the internal and external strategical considerations mentioned in REFERENCE PARAGRAPHHHHHH!!!!!!!!. The duration of this phase is around 2 years.
Preclinical Development: The preclinical testing focuses on basic pharmacology, toxicology and drug metabolism. This phase helps to gather sufficient information to secure clinical trial application required for clinical studies in humans. The outcome is Investigational Exemption for a New Drug (IND), necessary to be allowed to proceed with clinical testing in humans. On average this phase lasts 2 to 4 years and the laboratory experiments are in vitro and/or animals following the Good Laboratory Practice (GLP).
Phase I: this phase studies initial safety and tolerance evaluations. It establishes the pharmacokinetic profile and it provides information about the efficacy of the new drug in healthy human volunteers.
Phase II: in this phase the drug is tested on volunteer patients with the disease the drug aims to cure. The drug is tested in different dosage ranges and administration frequencies. It aims to demonstrate the efficacy of the drug in different dosage ranges. The outcome is a Proof of Concept (POC) that allows continuing the studies in Phase III based on appropriated dosage ranges and administration frequency.
Phase I and Phase II together take around 2 to 4 years to be accomplished.
Phase III: is the large-scale program consisting of several tests in a thousand of patients. Consists of several double-blind, placebo-controlled clinical studies to demonstrate efficacy and safety of a selected dose and dosing frequency. During this phase the application to market (NDA or MAA) is made and data and information to support the product launch are under review by authorities.
Phase IV: this post-approval phase is conduct to gather more data regarding efficacy and safety in real-practice conditions in a large scale and broad patient population. These studies conduct usually to the creation of a lot of data used for scientific publication and physician education.
Life Cycle Plan: in parallel with Phase III and Phase IV, activities related to the Life Cycle Plan (LCP) start. The Life Cycle Plan is a long-range strategic plan that describes all potential additional development activities that will extend and maximize the value of the drug through its period of market exclusivity. This initiatives can be grouped into the following major categories:
- Development initiatives: diseases or conditions for which the drug’s mode of action is applicable and where currently sufficient unmet medical needs exist.
- Drug modification: new formulations, dosage forms , or combinations with other product to improve the product profile;
- Geographic initiatives: expanding clinical programs and regulatory activities to secure market authorizations in additional countries;
- Manufacturing and distribution initiatives: activities related to the reduction of the cost of goods.
Project management processes in pharmaceutical R&D
By applying project management discipline, pharmaceutical companies are able to achieve efficiencies, minimize costs and maximize time savings. Project management can also contribute to process improvement and optimization, as well as contribute significantly to the overall success of drug development project by virtue of its coordination and operating oversight roles.
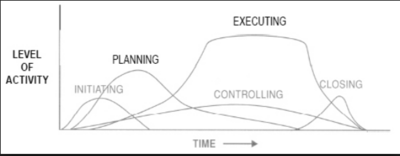
Project management team manager follows a set of interrelated actions in order to provide the desired deliverable, and a good practice and framework is given by the PMBOK guide where the five categories known as Project Management Process Groups are described as follows:
- Initiation: ¨Processes that define a new project or a new phase of an existing project and the authorization to initiate the project or phase
- Planning: Processes to develop the scope of the project that clarifies future decision-making in order to attain the objectives.
- Execution: Processes initiated in order to complete the work
- Controlling: Processes to to maintain, review and regulate the progress
- Closing: Finalizing all activities and closing the project
Initiation In this Process Group the project manager, if not already assigned, will be selected. Then the initial scope and the financial resources are defined together with a stakeholders analysis to identify internal and external stakeholders. For large and complex projects as a R&D project is, the project should be divided into separate phases. Each of this phases should go through the initiating processes to validate the decision made during the original development. Success criteria need to be defined in order to evaluate whether a project should be continued, delayed, or discontinued.
Planning The project managements team has do refine the objectives and scope of the Initiation and develop the course of action required to attain those objectives. Due to the complexity, uncertain and length of R&D projects, the use of repeated feedback loops for additional analysis is required. Moreover, it is important to define the level of details that wants to be achieved: continuos changes occurring in the execution of the project force to revisit one or more planning processes and they might require to revisit some of the initiating processes as well.
Execution This phase aims to complete the work defined in the first two phases to satisfy the project specifications. It is therefore required coordination of people and resources, management of stakeholders as well as performing the activities of the project in accordance with the plan made. During the execution there may be some unexpected changes in duration, resource productivity or availability, and unanticipated risks that require a reformulation or update of planning processes.
Monitoring and Controlling These processes require to maintain, review, and regulate the progress and performance of the project, identifying area in which changes are required. In complex projects, the Monitoring and Controlling Process Group also monitors and coordinates project phases in order to implement corrective and preventive action to avoid off-roads of the project itself.
Closing Consists of those activities necessary to conclude all activities across the all project to formally conclude it. The project can be conclude because successful, and it therefore establishes that the planned processes have been completed within budget and time constraints. But it may prematurely close a project due to many factors linked to the drug or to authorities that come up with new regulations.
Approaches in R&D project management
Project management provides necessary best practice, but different companies can choose among varied approaches. The three that are going to be analysed are:[5]
- Many working as One – distributed project management system;
- Matrixed structure – centralized governance;
- Recruiting of staff with project management experience to lead its efforts – recommended for smaller company.
Many working as one
Due to the high number compounds and conditions to address, a pharmaceutical company might need many project managers. But rather then centralize the project management function in a single office, the company organizes project management staff in such a way to ensure to have a lot of project management groups across various function. Within the same group there are people from different departments collaborating for the same goal. The different groups may vary in number of people and phases covered based on the size of the company and on the progress of the company of implementing this approach. Although the project management teams are not centralized, there is a central repository of knowledge to help guide project teams and keep everyone on the same page.
Surely an efficient way of working that allows to get information and proceed with choice in a more agile way. The teams are more empowered and there different background of the team members allows a smoother flow of information. Decentralizing the teams, though, might bring to less focus on the overall company strategies and goals, making the team members more loyal to their team rather than the company itself. Moreover, a lack of communication between different groups may lead to the repetition of same projects.
Matrixed structure
In contrast with the first one, this approach angles for a more centralized project management office. All the projects therefore start from the centralized office. Then people are assembled into teams for specific projects and the central office provides regular training to teach staff the project management. Doing so the company oversees projects to make sure the employees stick to good project management principles. This approach is mainly used to avoid the lost of centrality in the processes – often the people in charge of a project team would get moved or transferred within the company or would leave the company without no one else knowing what was going on with the project. Or project would be open but going nowhere and never close, wasting resources. This allows to educate people about the importance of project management principles building up a project management culture in the organization.
This different systematic way of proceeding allows a better understanding of the project management principles for the employees. It has some critical factors if not executed in the best way, though:
- Pharmaceutical projects may not be estimated correctly. Request for project in pharmaceutical R&D are dealt as they come in, with rough estimation. This might require to much time spent to propose a project that might be denied afterwards;
- Project managers might not have the competencies to evaluate the complexity and good prospective of the proposed project.
Setting the stage
This kind of approach is suggested for smaller companies that want to work toward a project management culture. Even though the company is not large enough to warrant establishing a formal group, it does incorporate the essential tools. This practice is really suggested for small company in which planning ahead is critical. This is why hiring experienced people in the field is fundamental, they have the required tools and skills to make a general map, they know where they are and when it is time to quit a project.
This three different approaches have different pros and cons but generally, if executed in the right way, can surely bring more advantages to the company. It is then the company itself that, based on size, organization and prerequisites decides whether to go for one approach or for another one.
Project Manager: role and activities
The role of the project manager in pharmaceutical R&D is a critical role that requires both technical and soft skills. It must be able to cope with projects that range in duration from six months to ten years or more. And while focusing on making the project successful, it also needs to be aware of the organization’s strategical goals. In the following section the level of technical and management skills and the day to day activities are analysed.
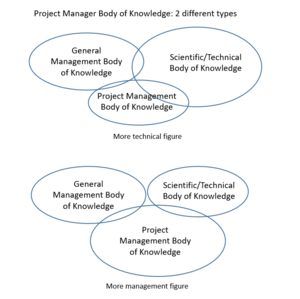
Body of Knowledge
The body of knowledge (BoK) is the complete set of concepts, terms and activities that make up a professional domain: it thus defines a profession as a knowledge representation.[5] The BoK representing the role of the project manager is usually equally balanced between General Management, Scientific/Technical, Project Management. For what concern the pharmaceutical R&D project manager there are some different opinions, mostly based on the technical competences required to cover such a role. On the other hand, though, due to the complexity and length of the project its role requires a lot of knowledge in the project management field. Planning and breaking down the activities in a good way can determinate a success or a failure in the project.
In the first case, the supporter of this type of project manager point out the fact that it has to have more Scientific/Technical BoK – in fact it has to manage and understand the interdisciplinary scientific issue arising around the discipline of pharmacology, chemistry, toxicology, metabolism, pharmacokinetics, pharmacy, clinical research, and statistics.[5] This represents one case and, obviously, the higher the two competencies are, the better it is.In the other hand, the project manager is purely seen as a coordinator, scheduler, and operations researcher. This brings some useful strategy in an organization because some scientist feel less threatened by non-scientists’ asking question – they appear less judgmental.
The truth should be in between, finding a trade off between the two different opinions:any project manager is probably positioned somewhere in between those two poles.
Day-to-day activities
The project manager has to deal with the project since its born until its completion. In fact the role itself is decided in the Initiation and it falls in the Closing. In addition to the activities described in the REFERENCE PARAGRAPH ABOUT 5GROPUS it has day-to-day activities to deal with, ensuring the all process is going towards the direction agreed. In this scenario the rule of the project manager will be more team-facing, maintaining alignment between commercial and technical strategies, directing team operations, and assuring to keep up-to-date information relative to ongoing activities, budget, and future plans. The day-to-day activities of the project manager can be listed in 5 different groups:[4]
- Ensure strategic and operational alignment: it defines and ensures that commercial and technical objectives are aligned. This alignment is important to ensure that the decision-making process is inside the strategic context
- Planning and execution: it is responsable for key planning tasks, creating detailed project plans for the daily activities. Moreover, it monitors key milestones, adjusting plans, if necessary, in order to delivery on schedule with the fixed budget
- Risk management: it ensures that the risks are taken on time and that the risks contingency are communicated to the stakeholders in the right moment to give them time to react
- Communication: it manages communication exchanges between the stakeholders
- Team effectiveness: it manages team interactions, leads the meeting and strive the team to give the best.
Stakeholders
Due to the length of the project the stakeholders change over time and they are both internal and external. It is really important to keep a good and transparent communication with them in order to minimizing the risks: in fact, information from one functional group impacts progress of another functional group. Proactive communication should be encouraged at every level to prevent any confusion and unnecessary rework.
References
- ↑ 1.0 1.1 "Pattanaik, A.Complexity of project management in the pharmaceutical industry. Paper presented at PMI® Global Congress 2014—EMEA, Dubai, United Arab Emirates. Newtown Square, PA: Project Management Institute. - Available at: https://www.pmi.org/learning/library/project-management-complexity-pharmaceutical-industry-1487 "
- ↑ "Pharma Figures 2018 Report - Available at: https://www.efpia.eu/media/361960/efpia-pharmafigures2018_v07-hq.pdf/EFPIA"
- ↑ "What goes into the cost of prescription drugs? Pharmaceutical Research and Manufacturers of America. Washington, DC . June 2005 , 15.)"
- ↑ 4.0 4.1 4.2 "D. L. Raemdonck & B. A. Burns, Drug Development project management. Chapter 4. Available at: https://onlinelibrary-wiley-com.proxy.findit.dtu.dk/doi/pdf/10.1002/9780470636930.ch4""
- ↑ 5.0 5.1 5.2 Dowden, S. B. (1995). The project manager in pharmaceutical R&D. PM Network, 9(5), 15–22. - Available at: https://www.pmi.org/learning/library/project-manager-pharmaceutical-research-development-4975