Corrective and Preventive Actions (CAPA)
Contents |
Abstract
Corrective and Preventive Actions (CAPA) are procedures designed to handle nonconformity and other undesirable situations. In this context is corrective actions is defined as actions set in motion to eliminate an occurred nonconformities or unwanted situations, and preventive actions as actions set in motion to eliminate potential nonconformities or unwanted situations [1]. CAPA is a mandatory part of the Quality Management System (QMS) for any pharmaceutical or medical device manufacturer reporting to the U.S Food and Drug Administration (FDA). CAPA is also an integrated part of ISO:13485 and Good Manufacturing Practice (GMP) for medical products. The FDA defines the purpose of a CAPA procedure as: collecting and analyzing information, identifying and investigating product and quality problems, and taking appropriate and effective corrective and/or preventive action to prevent their recurrence [2]
The purpose of this article is to give the reader an overview on how to perform a CAPA and which risk to be aware of. In this article a 7 step framework is presented. The steps include 1) Identification; 2) Evaluation; 3) Investigation; 4) Analysis; 5) Action Plan; 6) Implementation; 7) Follow-up.
The context of CAPA
Project quality management
A project is an unique temporary organizational construction with a defined objective. Projects can operate under different constrains such as time, cost, quality, etc. CAPA projects are related to quality management of a product/system. Quality management is the coordinating of activities to direct and control an organization with regard to quality. The CAPA process can be used as a quality management approach when working with project management. A quality management approach is a procedure that describes how quality will be managed in a given project [3].
Continuous improvement
CAPA is categorized under the section measurement, analysis and improvement in ISO:13485 and closely related to continues improvement processes. CAPA can be an alternative to processes such as the plan-do-check-act (PDCA) by Shewhart (modified by Deming), or other quality improvement initiatives such as total quality management (TQM), Six Sigma, and Lean [3].
Regulatory authorities
CAPA is most commonly used in highly regulatory industries and is a mandatory part of a Quality Management System (QMS) for any pharmaceutical or medical device manufacturer reporting to the U.S Food and Drug Administration (FDA) or who is compliant with ISO:13485 Medical Devices as well as European Pharmaceutical GMP and IATF. Procedures for corrective actions is also mandatory to include in the QMS for the majority of ISO management systems.
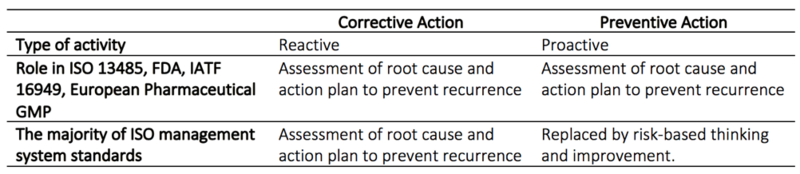
The purpose of an efficient CAPA process
CAPA is designed to ensure quality and continuous improvement, which is especially important in the pharmaceutical and medicine device industry since lack of control can have fatal consequences. CAPA is one of the most essential regulatory area for both the FDA and ISO in pharmaceutical quality systems and industries producing medical devices [2], [5]. The CAPA system is practically always a part of the regulatory compliance audit and around 30-50 percent of nonconformance cited by the FDA is due to inadequate performance of CAPA [5]. In the worst case can the FDA withdraw their certification, which is necessary to sell production on the U.S market. The CAPA process is more demanding and rigid than other similar approaches such as the PDCA. This is to ensure that all regulatory demands are fulfilled [1].
The 7 steps of CAPA
The CAPA process is typically divided into 5-9 steps depending on the scope of the steps. In this article a 7 step framework is introduced. The steps include 1) Identification; 2) Evaluation; 3) Investigation; 4) Analysis; 5) Action Plan; 6) Implementation; 7) Follow-up. When CAPA is performed as a part of a regulatory requirement such as FDA or ISO:13845 documentation through each step is crucial.
Step 1: Identification
The first step of the CAPA process is to identifying, describe and document the problem. A nonconformity incident does not necessarily trigger a CAPA. However it is necessary to evaluated every nonconformance and assess what action is appropriate. Nonconformance can be identified from both internal and external sources, including, but not limited to:
- service request;
- internal quality audits;
- costumer complaints;
- quality assurance inspections;
- staff observation;
- trending data;
- risk assessment;
- management review;
- failure mode analysis;
- Audit findings [6]. [7].
When a problem is discovered, a clear problem statement should be written. It is important to precisely and completely describe the situation. The problem statement should include the source of the information, evidence of the problem and a detailed explanation of the problem [6]. Here answering the following questions can be helpful.

Information about the problem will often be provided form different parts of the organization, and it can therefore be helpful to develop a standard procedure for collecting this data, and trying to reduce the work load for the employees[8]. A well-defined problem statement is essential for the later work and the effectiveness of the CAPA process [5]. As the cited quote from Charles Kettering emphasizes “a problem well-stated is a problem half-solved”.
Step 2: Evaluation
In step 2 the problem is evaluated to determine why the problem is a concern, and the impact and risk associated with the problem. This can be the possible impact on costs, function, product quality, safety, reliability, customer satisfaction, etc. [5]. The risks can be evaluated based on the probability/impact matrix.
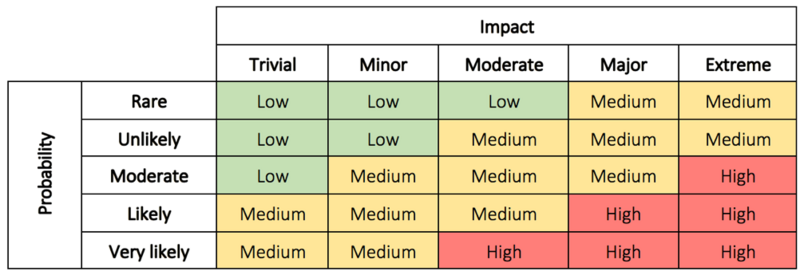
The evaluation is used to decide the priority of the actions as well as the level of investigation. It makes it possible for management to better prioritize encountered complaints, deviations, nonconformance, corrections, and removals [5]. In some cases immediate corrective action is necessary until a thorough investigation have been conducted and permanent solutions have been implemented [6]. The immediate corrective actions can in some cases be enough to correct the problem which eliminate the need for step 5 and 6 [6]. In other cases, it is assessed that no action is required. If this is the conclusion a justification must be documented.
Read more about impact and probability in risk assessment here
Step 3: Investigation
In step 3 a procedure for the investigation (analysis) if formulated. High-risk investigations will have priority over low-risk situations [2]. The purpose of the procedure is to ensure that noting is overlooked in the investigation. The procedure should include information about, but not limited to: the objective for the actions, which resources is required (money, time, testing equipment, personnel, etc.), who is responsible for conducting the investigation, which data is collected, and instructions for determining the causes of the problem including a timeline [5], [6]. The procedure require a review of all circumstances related to the problem and must consider the following:
- External factors
- Software
- Training
- Design
- Procedures
- Personnel
- Materials
- Equipment
The level of investigation is determined based on the risk evaluation results or trend analysis results [5].
Step 4: Analysis
Step 5: Action plan
Step 6: Implementation
Step 7: Follow-up
CAPA Report and documentation
Limitations
Critically reflect on the tool/concept/theory and its application context. What can it do, what can it not do? Under what circumstances should it be used, and when not? How does it compare to the “status quo” of the standards – is it part of it, or does it extent them? Discuss your article in the context of key readings / resources provided in class. Substantiate your claims with literature
Other relevant Wiki articles
How to create a working CAPA team: Roles and responsibilities in project team.
How to control the scope of the CAPA project: Project Scope Management.
How to ensure control of the CAPA proces: Project Control.
How to improve your CAPA processes: Lessons learned.
Annotated Bibliography
Provide key references (3-10), where a reader can find additional information on the subject. The article MUST make appropriate references to the and reference material provided in class – either incorporating it as a source, or critically discussing aspects that are missing from it but covered by this article. Summarize and outline the relevance of each reference to the topic (around 100 words per reference). The bibliography is not counted in the suggested 3000 word target length of the article.
References
- ↑ 1.0 1.1 Rodríguez-Pérez, José American Society for Quality 2011, CAPA for the FDA Regulated Industry
- ↑ 2.0 2.1 FDA website Corrective and Preventive Actions (CAPA). Link: https://www.fda.gov/corrective-and-preventive-actions-capa#page3.
- ↑ 3.0 3.1 AXELOS, Managing Successful Projects with PRINCE2 2017 Edition chapter 8
- ↑ Hammar, Mark Complete guide to corrective action vs. preventive action. Link: https://advisera.com/9001academy/blog/2020/06/22/complete-guide-to-corrective-action-vs-preventive-action/
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Rodriguez, Jackelyn. CAPA in the Pharmaceutical and Biotech Industries – How to Implement an Effective Nine Step Program 2016, Woodhead publishing series in biomedicine
- ↑ 6.0 6.1 6.2 6.3 6.4 Simplifying CAPA: Seven Steps to a Comprehensive CAPA Plan 2019 MasterControl. Link: https://www.mastercontrol.com/learning-center/capa-management/simplifying-capa-seven-steps.
- ↑ Abhishek, Raj. 2016 A review on corrective action and preventive action (CAPA) Deputy R&D Manager, Quest Pharmaceuticals Pvt. Ltd, Chhata Pipara, Bara, Birgunj, Nepal. Link: https://academicjournals.org/journal/AJPP/article-full-text-pdf/4EF704756791
- ↑ 8.0 8.1 Okes, Duke 2009. Root Cause Analysis – The core of Problem Solving and Corrective Action. ASQ Quality Press.